
(Kerry loves this little nerd, so asked for it to be included in my post.)
First, I need to say a bit about how atoms (specifically, their nuclei) are put together. This little page has a great primer on nuclear energy in general, and I'll be cribbing from it liberally.
Nuclei are made up of protons and neutrons. These are both relatively massive particles in the atomic world, but the mass of a nucleus is always less than the sum of the individual masses of the protons and neutrons which constitute it. The difference is a measure of the nuclear binding energy which holds the nucleus together. This binding energy can be calculated from the Einstein relationship, which you've seen before. E=mc^2. Only this time, the "m" is a difference in mass between the sum of the individual masses of the protons and neutrons and the actual mass of the nucleus, and E is the energy that holds those neutrons and protons together.
Here is a plot of the binding energy vs nuclide mass or number of nucleons in the atomic nucleus. Nickel 62 is the most tightly bound nuclide. You'll often see it claimed, incorrectly, that Fe56 is the most tightly bound nuclide. This comes from astrophysics where Fe56 is the most common stable nuclide produced in stellar nucleosynthesis processes.

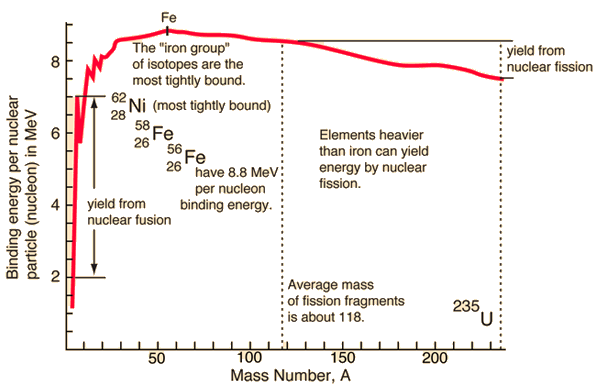

So, as you can see in the plot, you can release the nuclear binding energy by one of two processes. Fission is the process in which you break apart a nucleus of a heavy element (Uranium, for example), creating lighter elements and energy, with some possible other particles released as well. Fusion is the process in which you push two light nuclides together until they form a new element, releasing a bunch of energy. Fusion happens in stars, generating helium and heavier elements from hydrogen. Fission happens in nuclear bombs and nuclear power plants. Fission also happens naturally. We cannot yet get more energy out of a fusion event than we put in, so I will only speak about fission. Interestingly, we can get quite a bit more energy from fusion than from fission, but we need incredible amounts of energy to control the reactions; once we can figure out how to cheaply create the environment at the center of the sun, we can have much, much, much more than enough cheap and "easy" energy... Oh, wait, we have such an environment only 1 AU from us... ;)
Nuclear fission can happen with elements heavier than those in the "iron group", which includes Fe56 and Ni62. The very basic idea is that a slow moving neutron is collided with a massive nuclide (say, Uranium 235). During this collision, Uranium 235 absorbs the nuclide and its energy. Suddenly the nucleus has more energy than the binding energy required to hold it together. This means that there's enough energy to break the nucleus apart. The products are, in one instance, Rb90 and Cs143, a few neutrons, and a bunch of energy. Rb90 and Cs143 are unstable, with half-lives on the order of minutes to seconds; we'll talk about that next time.
Notice that the mass of Rb90+Cs143 is not 235. That missing mass is mostly in the form of energy (about 200 million times as much energy as the slow moving neutron had). There are a few more neutrons as well, but not enough to account for the missing mass. We'll talk about those neutrons in a bit, but first we have to explain "slow moving" neutron.
We need a slow moving neutron because a fast moving neutron won't be captured. In order to control the speed of the neutron, we use something called a neutron moderator. The most common moderator is light water (doesn't have deuterium). That's what is meant by a "light water reactor". There are a number of other moderators in use, and several being researched. These moderators produce what we call "thermal" or "slow moving" neutrons from faster neutrons.
Back to those newly produced neutrons from the neutron+U235 collision. We can use those neutrons to collide with other U235 nuclei, giving us a chain reaction. If we can control this reaction by only allowing one (no more, no less) neutron from each collision to go on to another collision, we can have a sustained but controlled nuclear fission reaction. We control the number of neutrons allowed to participate in collisions by means of the control rods, usually made of boron or cadmium. These elements can absorb many neutrons without themselves becoming unstable. The control rods could get too hot, which would cause them to melt and stop being effective in controlling the chain reaction, causing something like the Three Mile Island accident (like, not identical to).
If we have fewer than one neutron going on to collide with more uranium, the reaction dies and everything stops. No energy generation. If we allow more than one neutron to collide with more uranium, we have an uncontrolled chain reaction with a positive feedback loop, very quickly releasing a lot more energy than we could possibly control in the reactor. This is basically what nuclear bombs do, but it's a lot more complicated, depending on the way things happen.
To maintain the temperature of the control rods, the core, and other components of the reactor, water is used as a coolant. The energy released is so much that the water is quickly converted to steam. This steam is used to drive turbines. The steam-driven turbines generate the electricity, much like coal- or natural gas-fired plants steam-driven turbines.
This has been a very simple and basic description of the process by which nuclear power is used to generate electricity. I'm going to have to add another post or two to discuss the pros and cons and other elements to nuclear energy.




3 comments:
This is a really good post, and not just because it has the funny guy with the eyebrows : ). I was happy to discover that I even remember some of this from high school chemistry--yay Mrs. Flaccus!
I, too, love the eyebrows.
"Oh, wait, we have such an environment only 1 AU from us" - you mean the sun, right? I know you're being witty, but I have to struggle with this stuff. :-)
Of course I mean the sun. Did you click the link? ;)
Post a Comment